ISSUE1746
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of subcutaneously injected, extended-release risperidone (Uzedy) for treatment of bipolar disorder.
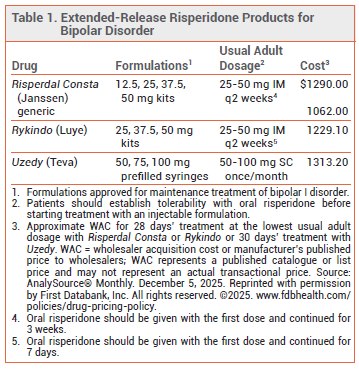
Uzedy, an extended-release, subcutaneous (SC) formulation of the second-generation antipsychotic drug risperidone, was approved by the FDA in 2023 for treatment of schizophrenia in adults. It has now been approved for use as monotherapy or in combination with lithium or valproate for maintenance treatment of bipolar I disorder in adults. Extended-release, intramuscular (IM) formulations of risperidone (Risperdal Consta and Rykindo) are also approved for treatment of bipolar I disorder

MAINTENANCE TREATMENT — Lithium, used alone or in combination with another drug, is generally considered the drug of choice for maintenance treatment of bipolar disorder. Valproate, lamotrigine, and carbamazepine are alternatives. Second-generation antipsychotic drugs are effective in preventing recurrences of manic and depressive episodes, especially when taken in combination with a mood stabilizer (lithium or valproate); they may be more effective in preventing hypomania than depression. Extended-release injectable second-generation antipsychotic drugs are generally used in patients with a history of relapse due to poor adherence to oral maintenance therapy; they are believed to reduce hospitalization and relapse rates.2
PHARMACOLOGY — Unlike with IM formulations of risperidone, absorption of risperidone is rapid following administration of Uzedy, eliminating the need to continue taking oral risperidone for the first several days or weeks after the first injection to maintain therapeutic levels of the drug. Serum concentrations of risperidone with Uzedy are maintained for 4-8 weeks post-injection. Serum concentrations of risperidone are maintained for 2-4 weeks with Rykindo and for 4-6 weeks with Risperdal Consta.
CLINICAL STUDIES — No new clinical efficacy trials were required for FDA approval of Uzedy for the new indication; approval was based on the results of earlier trials in patients with bipolar I disorder who were treated with long-acting formulations of risperidone that were given intramuscularly once every 2 weeks.
ADVERSE EFFECTS — Subcutaneously administered risperidone can cause injection-site reactions, akathisia, angioedema, dyslipidemia, tremor, parkinsonism, hyperglycemia, hyperprolactinemia, weight gain, and rarely priapism. Seizures can occur in patients with a history of seizure disorder.
CONCLUSION — Uzedy, an extended-release, subcutaneously administered formulation of risperidone approved for maintenance treatment of bipolar I disorder, extends the interval between injections from every 2 weeks with intramuscular risperidone formulations to once monthly. Unlike with intramuscular formulations of risperidone, Uzedy does not require concomitant administration of oral risperidone after the first injection to maintain therapeutic levels of the drug. Uzedy does not appear to offer any advantage in efficacy or safety over other injectable formulations of risperidone.